Commitment to papaya soap
Difference between soap and synthetic detergents
Do you know how sweat and sebum “dirt” is cleaned off?
A compound that can clean off dirt created by combining oil and water, two materials that normally cannot be mixed, is called a surfactant. Surfactant include compounds made by combining naturally-derived raw materials such as soy saponin and the lecithin in egg yolks with caustic soda (sodium hydroxide) or caustic potassium (potassium hydroxide) as well as chemosynthesis compounds which are made from petroleum raw materials. Those made from the naturally-derived materials are soaps, and those made with chemosynthesis are synthetic detergents.

Soaps can be broadly classified into two categories: sodium soaps (bar soap) and potassium soaps (gel or liquid soap).
Fatty acids from plant or animal fats combined with sodium hydroxide (caustic soda) to trigger a reaction which generates “fatty acid sodium” to create sodium soap (bar soap). Potassium hydroxide (caustic potassium is added to fatty acids from plants or animals to generate “fatty acid potassium” to create potassium soap (gel or liquid soap). Fatty acid potassium is a highly water-soluble gel, and water can be added to it to convert it to a liquid. Fatty acid sodium and fatty acid potassium generated in this way are called “soap base,” and they are referred to as “soap” regardless of whether they are solid or liquid. Our papaya soap, “Blessing of Green Papaya,” is a bar type, meaning it is a sodium soap.
Hearing “sodium hydroxide” and “fatty acid potassium” may cause you to think “Is that a chemical?” “It’s not natural?” However, these are some of the few chemicals with proven safety, in other words, they are handled differently than chemical compounds that are harmful to humans. Also, it’s impossible to make soap without chemical reactions. Natto, yogurt, bread, wine, and alcohol are all the same way. These products are all the result of chemical changes driven by yeasts. Synthetic surfactants made from petroleum using chemosynthesis have effects on the body, so they must be handled carefully.

Characteristics of soap
1) When fatty acids are mixed with sodium hydroxide or potassium hydroxide to cause a chemical reaction, glycerin which separates from the fatty acids is released as a byproduct. This is the reason why additive-free soap is said to dissolve easily, but it also gives the soap a beneficial moisturizing effect. Soap gives off fatty acid ions in water. These fatty acid ions immediately transform into water-insoluble fatty acids and are adsorbed by the keratinaceous surfaces of the skin. When you rinse, they are not washed away, but instead remain on the surface of your skin, providing a moisturizing effect.
2) Since large amounts of separated fatty acids (glycerin) are adsorbed by the skin, the moisturizing effect is potent. The reasons soaps are more appropriate for use on the delicate skin of babies, atopic dermatitis, extremely dry skin, and extremely sensitive skin compared to synthetic detergents is that they provide a natural stimulus for the skin. られるのは、皮膚への刺激がは自然と備わっているからです。
Characteristics of synthetic detergents
1) Compared to soap, they provide stronger cleaning power and greater skin irritation.
2) Strong degreasing properties can result in removal of the sebum layer which forms the skin's natural barrier, causing dry and scaly skin.
3) Can break through the skin’s natural barrier, penetrating subcutaneously and damaging internal proteins.
4) Adsorb on the skin and hair, releasing moisture and causing dry skin.
5) Since some require a long time to be broken down by natural bacteria and others cannot be broken down at all, they can cause environmental degradation.
6) Although petroleum-based synthetic detergents have strong cleaning properties, they cause dry and scaly skin by destroying the skin’s natural barrier.
Moisturizing compounds are added to compensate for this, but the truth is, the majority of these moisturizers are chemical synthetic additives. Even if a product contains natural moisturizers, whether or not it is a synthetic detergent is an important point.
About the cold process manufacturing method
2 soap manufacturing methods: “saponification method” and “neutralization method”
There are two types of soap manufacturing, the saponification method used for additive-free soaps and the neutralization method, which uses machines to create large volumes of soap in a short time. Processing can also be categorized into two groups, “hot process,” the standard method with uses a heated kettle, and “cold process,” which does not heat the soap.
Saponification method
In the saponification method, a plant oil such as olive oil is combined with sodium hydroxide (caustic soda) to trigger a reaction and create a soap base. Also, highly water-soluble potassium soap is made by adding potassium hydroxide (caustic potassium) to fats to create a soap base.* Caustic soda and caustic potassium are essential for the soap manufacturing process, and with the saponification method, the natural drying process leaves almost residue. Glycerin also separates from the fatty acids and is released as a byproduct."
Neutralization method
Unlike the saponification method, the neutralization method is a manufacturing process that first separates fats into fatty acids and glycerin, then combines only the fatty acids with sodium for a faster chemical reaction. With this method, soap can be created in 3-4 hours, enabling mass production. Since the soap made this way doesn’t contain glycerin, it has powerful cleaning properties but is poor at protecting the skin. For this reason, finished soap often contains added glycerin, as well as a variety of other additives (preservatives, sequestering agents, antioxidants, foaming agents, pigments, fragrances, etc.).
Characteristics of the cold process and hot process (kettle heating method)
In addition to the two method categories above, saponification and neutralization, there are two different processes used to convert soap base into finished soap, differentiated by whether or not the soap base is heated.

Characteristics of the cold process and hot process (kettle heating method)
In addition to the two method categories above, saponification and neutralization, there are two different processes used to convert soap base into finished soap, differentiated by whether or not the soap base is heated.

Hot process (kettle heating method)
The soap base is mixed and heated to form the soap. Since the soap base is heated to temperatures in excess of 100℃ and processed over a period of several hours, heat-sensitive compounds are affected. For “Blessing of Green Papaya,” a heat-free method is required to preserve the papain enzymes and other cysteine enzymes contained in the fruit.
Reasons our papaya soap, “Blessing of Green Papaya,” is made using the cold process method
2) To protect the beautifying compounds in the olive oil
3) Fats are not degenerated or oxidized
4) The finished product contains large amounts of glycerin, a natural moisturizing compound
5) It gives the soap a gentle feel when used

Cold process method overview (how Blessing of Green Papaya is made)

1.Sodium hydroxide and purified water and carefully measured and mixed. Since sodium hydroxide releases heat when mixed with water, the solution is allowed to cool until reaches approximately 40℃.

2.The solution from step 1 is moved to a different container, the oil to be used is carefully measured, and heat adjustments are made so that this oil is also approximately 40℃.

3.When both parts are approximately 40℃, 1 and 2 are mixed together and continuously stirred for about 30 minutes. Next, the mixture is allowed to sit for a few hours until trace occurs (it thickens).
4.After trace, vegetable butters and essential oils are added and mixed in. Next, the mixture is poured into molds and left to rest at room temperature for 24 hours.

5.When the surface has hardened, it is removed from the molds and stored in a well-ventilated area to dry for one week.

6.Next, the hardened mixture is cut into single bars.

7.After cutting, the bars are lined up on pallets with some space in between them and aged and dried for approximately 60 days.
About “frame kneading”
Frame kneading and machine kneading methods
When the soap is complete, it is poured into a mold or other container to cool and dry over a long period of time. This is called frame kneading, and the finished product is cut into individual pieces after this process. With machine kneading, firm, well-shaped soap bars can be created in a short time, making mass production possible. Since this method makes soap production easy and fast, these products are sold at cheaper prices than additive-free soaps.

As the name suggests, machine-kneaded soaps use machines for every step of manufacturing process. The materials are kneaded rapidly by machines, then pressed into molds by machines as well. This enables mass production and low costs. Because these soaps are cheap with good lather and strong cleansing properties, mainstream hand soaps, bath soaps, and even general facial soaps are all machine-kneaded.
| Manufacturing method | Advantages | Disadvantages |
|---|---|---|
| Frame kneading |
|
|
| Machine kneading methods |
|
|
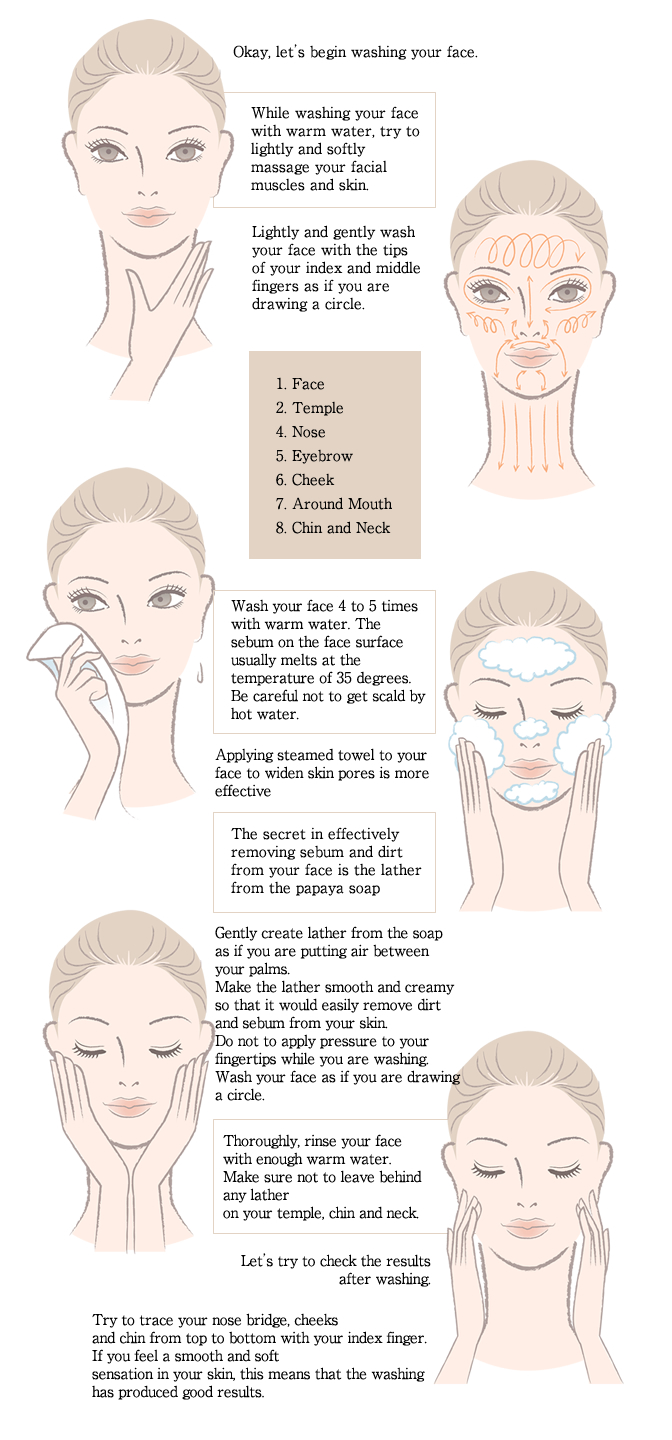
The proper way to use soap